1. Scope
This document outlines an HPLC method for determining xylo-oligosaccharide content in food products. It is suitable for detecting xylo-oligosaccharides in various types of food.
2. Normative References
The following documents are essential for the application of this method. For dated references, only the specified edition applies. For undated references, the latest version (including any amendments) applies:
- GB/T 6682: Specifications and Test Methods for Water for Analytical Laboratory Use
- GB/T 35545: Xylo-oligosaccharide
3. Terms and Definitions
The terms and definitions outlined in GB/T 35545 apply to this document.
4. Method Summary
Depending on the sample composition, this method involves extracting xylo-oligosaccharides from the sample using ethanol precipitation, zinc acetate, potassium ferrocyanide, or petroleum ether. The extract is hydrolyzed using sulfuric acid to yield xylose, which is quantified using HPLC. The difference in xylose content before and after hydrolysis, multiplied by a conversion factor, yields the xylo-oligosaccharide content. Quantification is performed using an external standard method.
5. Reagents and Materials
Unless otherwise stated, all reagents used are of analytical grade, and water meets the Grade 1 specifications as per GB/T 6682.
5.1 Reagents
- Acetonitrile (CH₃CN): Chromatography grade
- Anhydrous Ethanol (C₂H₅OH)
- Sulfuric Acid (H₂SO₄): Superior grade
- Sodium Hydroxide (NaOH)
- Ammonium Hydroxide or Triethylamine (NH₄OH or C₆H₁₅N)
- Petroleum Ether (boiling range: 30–60°C)
- Zinc Acetate [Zn(CH₃COO)₂·2H₂O]
- Potassium Ferrocyanide {K₄[Fe(CN)₆]·3H₂O}
- Glacial Acetic Acid (CH₃COOH)
5.2 Reagent Preparation
- 4.0 mol/L Sulfuric Acid Solution: Add 55 mL of sulfuric acid to 150 mL of water with constant stirring. Dilute to 250 mL with water.
- Sodium Hydroxide Solution: Weigh 20 g of sodium hydroxide, dissolve in water, and dilute to 100 mL.
- Zinc Acetate Solution: Weigh 21.9 g of zinc acetate, add 3 mL of glacial acetic acid, and dilute to 100 mL with water.
- Potassium Ferrocyanide Solution: Weigh 10.6 g of potassium ferrocyanide, dissolve in water, and dilute to 100 mL.
5.3 Standard
- Xylose Standard (C₅H₁₀O₅, CAS: 58-86-6): Purity ≥ 99% or certified standard material.
5.4 Standard Solution Preparation
- Xylose Stock Solution (5.0 mg/L): Weigh 0.125 g of xylose, dissolve in water, and dilute to 25 mL. Store at -18°C for up to 6 months.
- Xylose Working Solutions: Dilute stock solution to prepare working solutions with concentrations of 0.05 mg/mL, 0.10 mg/mL, 0.20 mg/mL, 0.50 mg/mL, 1.0 mg/mL, 2.0 mg/mL, and 5.0 mg/mL.
5.5 Materials
- 0.45 μm Filter Membrane: For aqueous solutions.
6. Instruments and Equipment
- HPLC System: With refractive index detector
- Electronic Balance: Sensitivity of 0.1 mg and 0.001 g
- Vortex Mixer
- Nitrogen Evaporator (Temperature Controlled)
- pH Meter or Wide-Range pH Paper
- Centrifuge: Speed ≥ 8000 rpm
- Thermostatic Water Bath
- Ultrasonic Cleaner
- Other Common Laboratory Instruments
7. Sample Preparation
7.1 Liquid Samples
Shake thoroughly, transfer to a sealed container, and refrigerate.
7.2 Semi-solid Samples
Homogenize the edible portion, transfer to a sealed container, and refrigerate.
7.3 Solid Samples
Grind to a uniform powder, transfer, and store at room temperature in a sealed container.
8. Analytical Procedure
8.1 Pre-hydrolysis Sample Preparation
- Liquid Samples: Take 5 mL of sample, add 10 mL of water, mix, add ethanol, centrifuge, and evaporate ethanol. Redissolve and prepare for HPLC analysis.
- Solid and Semi-solid Samples: Weigh 1-5 g, add water, sonicate, add ethanol, centrifuge, and prepare for HPLC analysis.
- High-Protein Samples (e.g., Dairy Products): Treat with zinc acetate and potassium ferrocyanide before adding ethanol and proceeding with further steps.
- High-Fat Samples (>10% Fat): Use petroleum ether to remove fat, followed by water extraction and ethanol treatment.
8.2 Post-hydrolysis Sample Preparation
Hydrolyze the sample with sulfuric acid at 100°C, adjust pH, and prepare for HPLC analysis.
8.3 Standard Curve Preparation
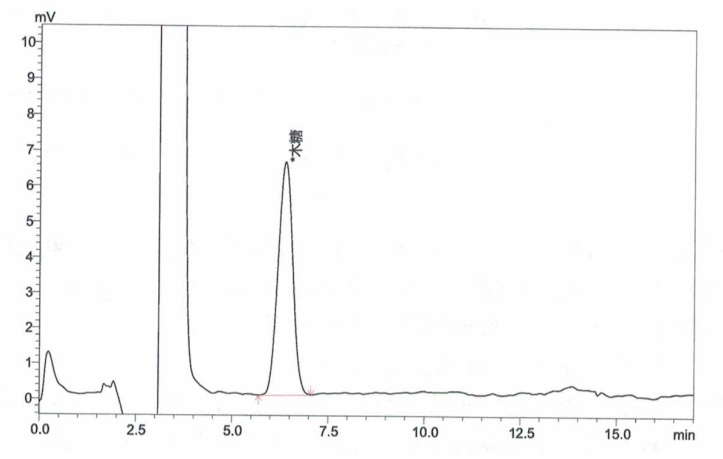
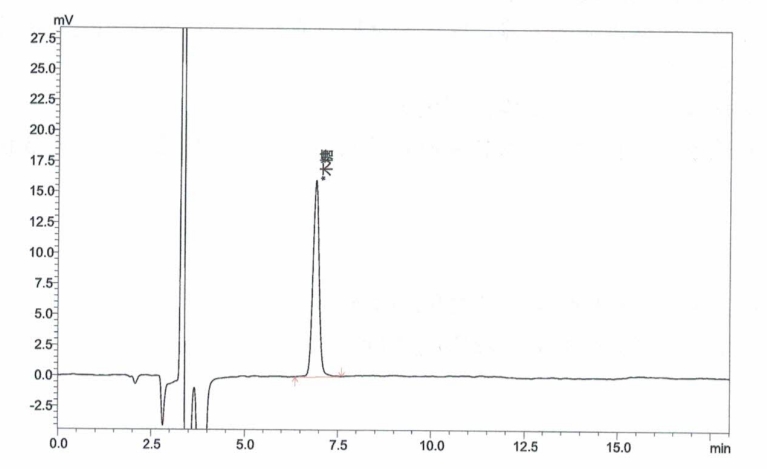
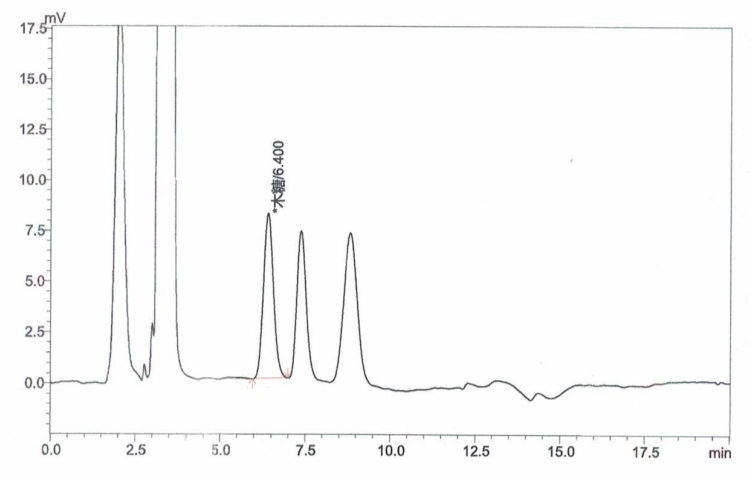
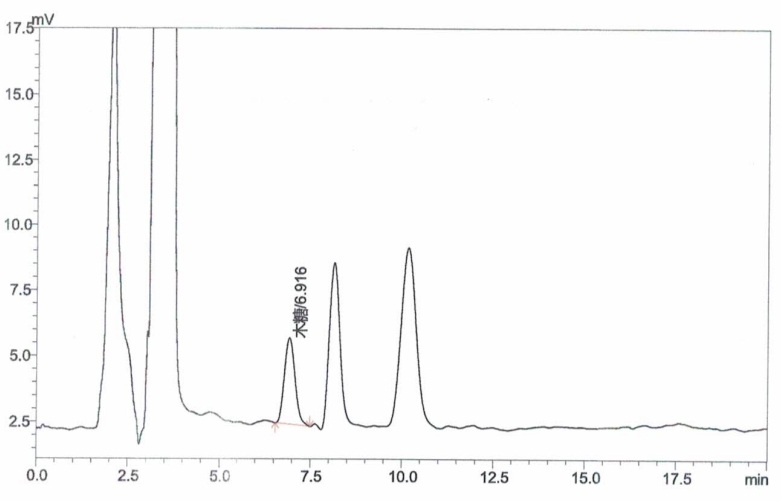
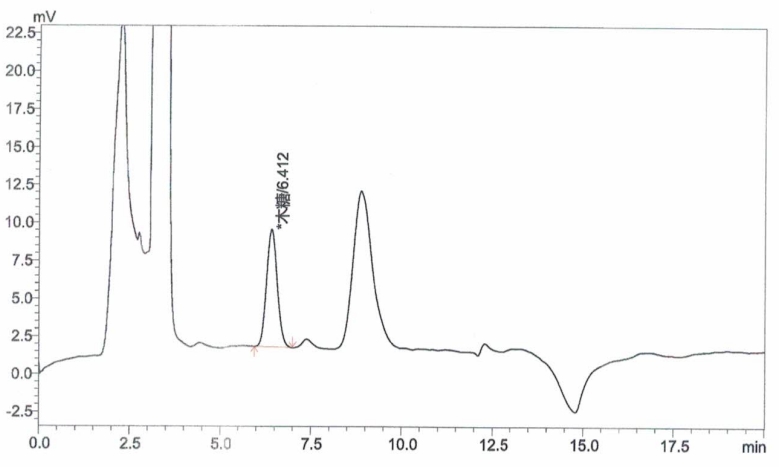
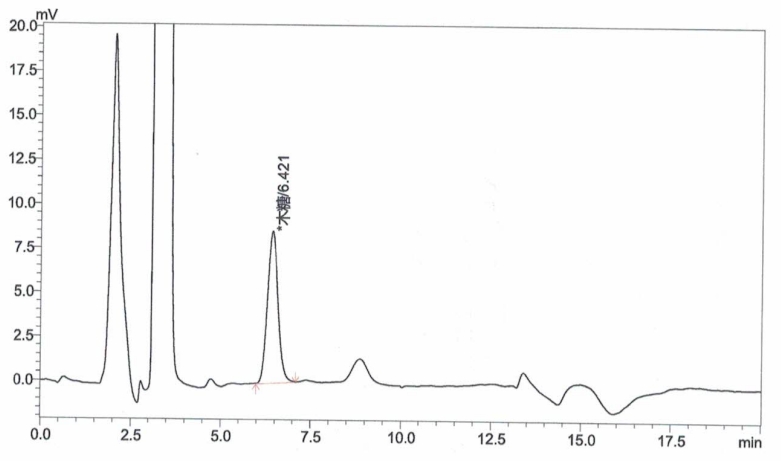
Inject xylose working solutions into the HPLC and plot a standard curve by peak area versus concentration.
8.4 Measurement
Inject the sample solution into the HPLC and measure the peak area.
9. Calculation of Results
Calculate xylo-oligosaccharide content using the provided equations, considering xylose concentration before and after hydrolysis, dilution volumes, and correction factors.

10. Sensitivity
- The detection limit for xylo-oligosaccharides is 0.1 g/100 mL for a 5 mL sample volume.
- The detection limit is 0.1 g/100 g for a 5 g sample mass.
11. Precision
The difference between two independent results obtained under repeatability conditions should not exceed 10% of the mean.
Appendix A: XOS Standard and Sample Chromatograms